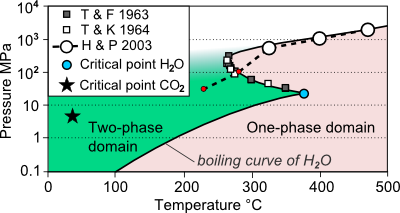
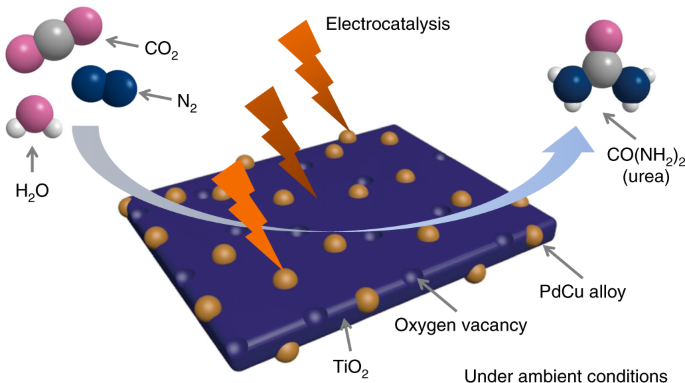
The promoting effects of CO2 and H2O on selective hydrogenations in CO2/H2O biphasic system - ScienceDirect

Write fully balanced equations for the following:(a) CO2 + H2O(b) Ca(OH)2+ CO2(c) SO2 + H2O(d) P205 + - Brainly.in

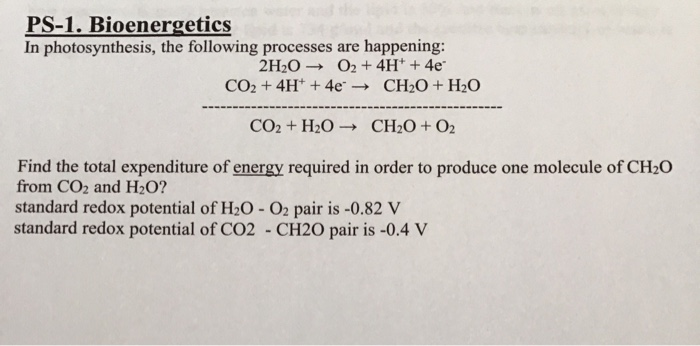
SOLVED: Photosynthesis: Light (energy) + CO2+H2O-> C6H1206+ 02+H2O Respiration: C6H1206 + 02–>CO2+ H20 + ATP (Chemical energy) These two processes are essentially the same reaction run in different directions. The main difference

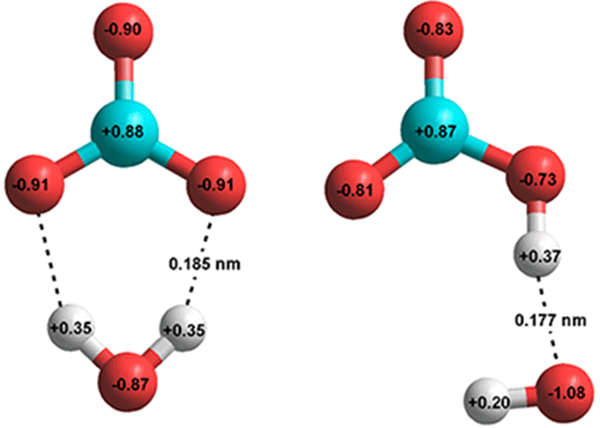
Water (H2O) and carbon dioxide (CO2) both have one central atom with two atoms bonded to it. However, one is a polar molecule and one is not. Draw the Lewis structure for

Utilization of H2O and CO2 in Coal Particle Gasification with an Impact of Temperature and Particle Size | Energy & Fuels
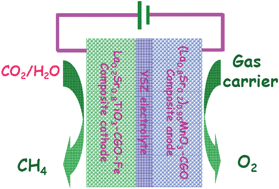
Direct synthesis of methane from CO2/H2O in an oxygen-ion conducting solid oxide electrolyser - Energy & Environmental Science (RSC Publishing)
![Identify Bronsted - Lowry acids in the given reaction. [ Al (H2O)6 ]^3 + + H CO3^- [ Al (H2O)5 (OH^-) ]^2 + + H2CO3 A B C D Identify Bronsted - Lowry acids in the given reaction. [ Al (H2O)6 ]^3 + + H CO3^- [ Al (H2O)5 (OH^-) ]^2 + + H2CO3 A B C D](https://dwes9vv9u0550.cloudfront.net/images/4940114/ca6cb326-72a5-4e75-8a33-8b87ccbb46e4.jpg)