Direct production of H2O2 from H2 and O2 in a biphasic H2O/scCO2 system over a Pd/C catalyst: Optimization of reaction conditions - ScienceDirect

Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte | Science
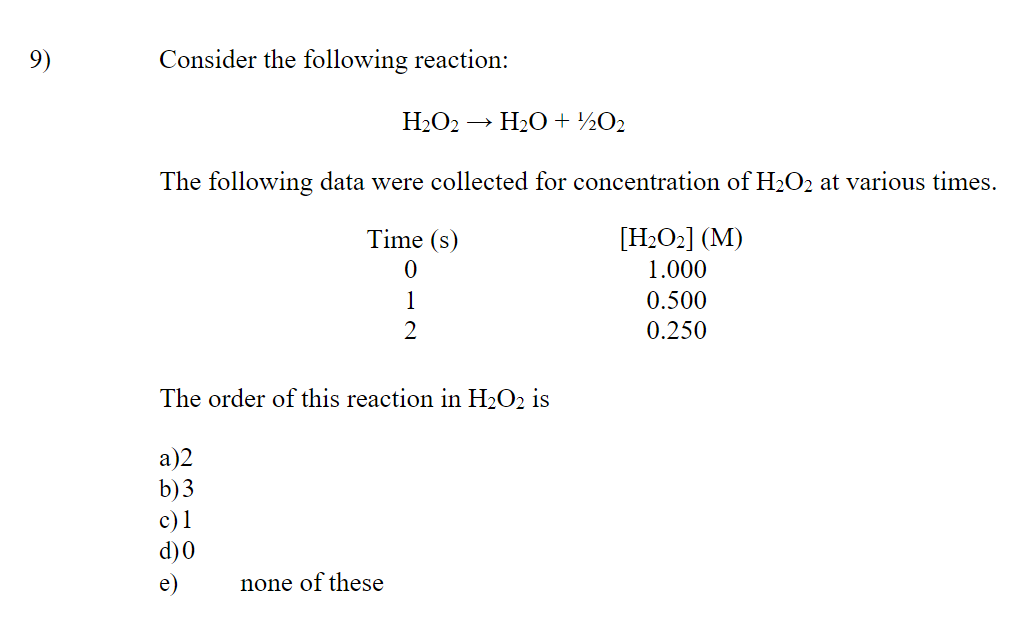
Why is the answer B? Can someone explain this to me and why other options are incorrect. I assumed that H2O2 will decompose rapidly to form H20 and O2 with MnO2 as

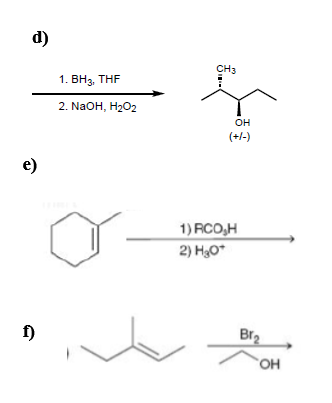
OneClass: Predict the product(s) of each of the following reactions: O NaOH, H2O, heat 1. LDA H 2. 1...
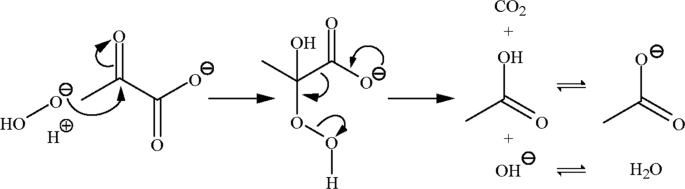
Reaction rate of pyruvate and hydrogen peroxide: assessing antioxidant capacity of pyruvate under biological conditions | Scientific Reports

Cells | Free Full-Text | Intracellular Sources of ROS/H2O2 in Health and Neurodegeneration: Spotlight on Endoplasmic Reticulum

The decomposition of H2O2 has a strong thermodynamic driving force 2H2O2→ 2H2O + O2(g) Δ H = - 99kj/mole,Δ s = + 69JK^-1 mole^-1 Addition of solution of KI causes H2O2 to

Amazon.com: 12% Hydrogen Peroxide (1 Gallon) Pure Food Grade H2O2 & Water - Made in USA - Ecofriendly BPA Free - Simple & Pure Cleaning Solution : Health & Household
![The crystal structure of trans potassium dioxalatodiaquochromiate, K[CR(C2O4)2(H2O)2].3H2O - Van Niekerk - 1951 - Acta Crystallographica - Wiley Online Library The crystal structure of trans potassium dioxalatodiaquochromiate, K[CR(C2O4)2(H2O)2].3H2O - Van Niekerk - 1951 - Acta Crystallographica - Wiley Online Library](https://onlinelibrary.wiley.com/cms/asset/29dbd8f8-5ed2-49f2-94d2-4332b7d55fcc/s0365110x51000064.fp.png)