Acid – Base Reaction. Chemical Reaction Neutralization The Acid And Base Properties, Producing A Salt And Water.

Trimyristin can be hydrolyzed in base NaOH to form myristic acid. Write a balanced chemical reaction. Trimyristin should be shown in line/bond form. | Homework.Study.com
Set Of Three Chemical Containers With Acid Base And Salt With Different Ph Hcl Hydrochloric Acid Naoh Sodium Hydroxide And Nacl Sodium Chloride Stock Illustration - Download Image Now - iStock

Sodium hydroxide (NaOH) is classified as a strong base. For every mole of sodium hydroxide added to a large volume of water, one mole of what ion enters the solution?

Vector Illustration Of Electrolytic Dissociation Molecules Break Up Into Ions Chemical Containers With Acid Base And Salt Hcl Naoh And Nacl Stock Illustration - Download Image Now - iStock



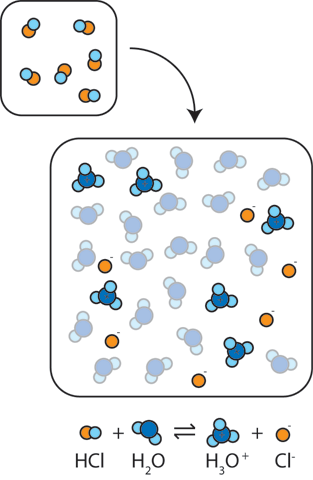

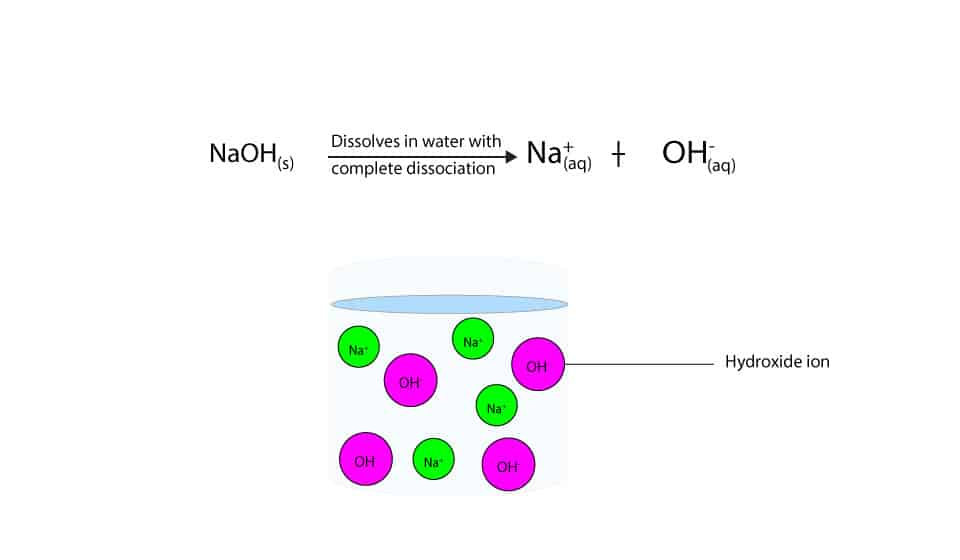

:max_bytes(150000):strip_icc()/prepare-sodium-hydroxide-or-naoh-solution-608150_FINAL-696b52d6f90b4b1383ec8f95db73a1f3.png)