30435-6X1KG - Natriumhydrogenphosphat dibasisch Dihydrat, reinst, zur Analyse, ≥98.5%, Plastikflasche, 6 x 1 kg analytics-shop.com
SODIUM DIHYDROGEN PHOSPHATE STARTING FROM SODIUM CHLORIDE AND ORTHOPHOSPHORIC ACID VIA CATION RESIN EXCHANGE Doan Pham Minh, A
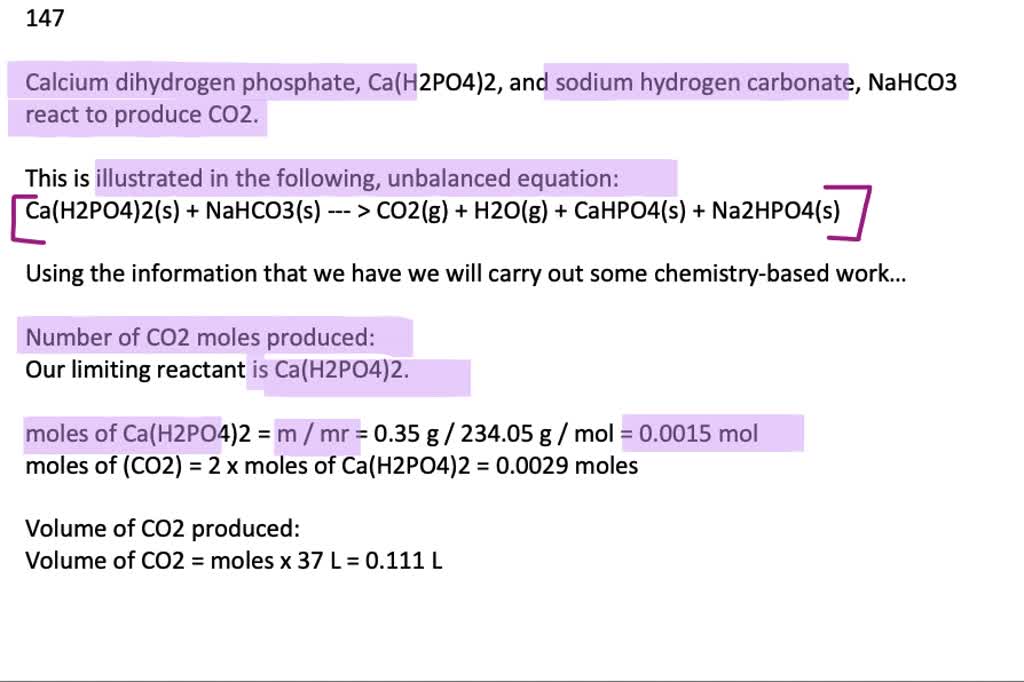
SOLVED: Calcium dihydrogen phosphate, Ca(H2PO4)2, and sodium hydrogen carbonate, NaHCO3, are ingredients of baking powder that react with each other to produce CO2, which causes dough or batter to rise: Ca(H2PO4)2(s) +

For the equilibrium SrCl2· 6H2O(s) SrCl2· 2H2O(s) + 4H2O(g) the equilibrium constant Kp = 16 × 10^-12 atm^4 at 1^0 C. If one litre of air saturated with water vapour at 1^0
![Sodium Phosphate Monobasic dihydrate (NaH2PO4.2H2O, 500g) [CN04-500G] - $20.00 : Bioland Scientific, for Your Research Needs Sodium Phosphate Monobasic dihydrate (NaH2PO4.2H2O, 500g) [CN04-500G] - $20.00 : Bioland Scientific, for Your Research Needs](https://www.bioland-sci.com/images/NaH2PO4s%20500G.jpg)
Sodium Phosphate Monobasic dihydrate (NaH2PO4.2H2O, 500g) [CN04-500G] - $20.00 : Bioland Scientific, for Your Research Needs

Equilibrium constants is given (in atm) for the following reaction 0^∘C : Na2HPO4. 12H2O(s) Na2HPO4. 7H2O(s) + 5H2O(g) ; Kp = 2.43 × 10^-13 The vapour pressure of water at 0^∘C is
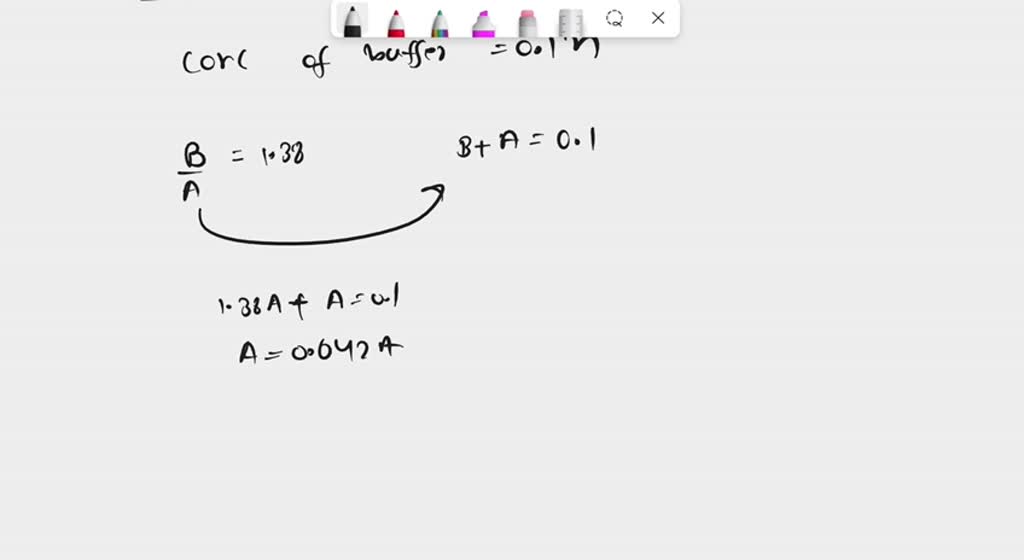
SOLVED: 1. How many g of Na2HPO4 and NaH2PO4 2H2O would you need to prepare 1L of 0.1M sodium phosphate buffer pH 7.0? (Hint= use the Henderson-Hasselbalch equation) Express your answer to