Jual Na2B4O7.10H2O Sodium Tetraborate Murah/Diskon/Limited/Berkualitas - Jakarta Selatan - JAMILLA_STORE | Tokopedia
![Sodium Borate Decahydrate [Na2B4O7.10H2O] 99.9% ACS Grade Powder 1 Lb in Two Space-Saver Bottles USA: Amazon.com: Industrial & Scientific Sodium Borate Decahydrate [Na2B4O7.10H2O] 99.9% ACS Grade Powder 1 Lb in Two Space-Saver Bottles USA: Amazon.com: Industrial & Scientific](https://m.media-amazon.com/images/I/91oK0hZSWxL.jpg)
Sodium Borate Decahydrate [Na2B4O7.10H2O] 99.9% ACS Grade Powder 1 Lb in Two Space-Saver Bottles USA: Amazon.com: Industrial & Scientific
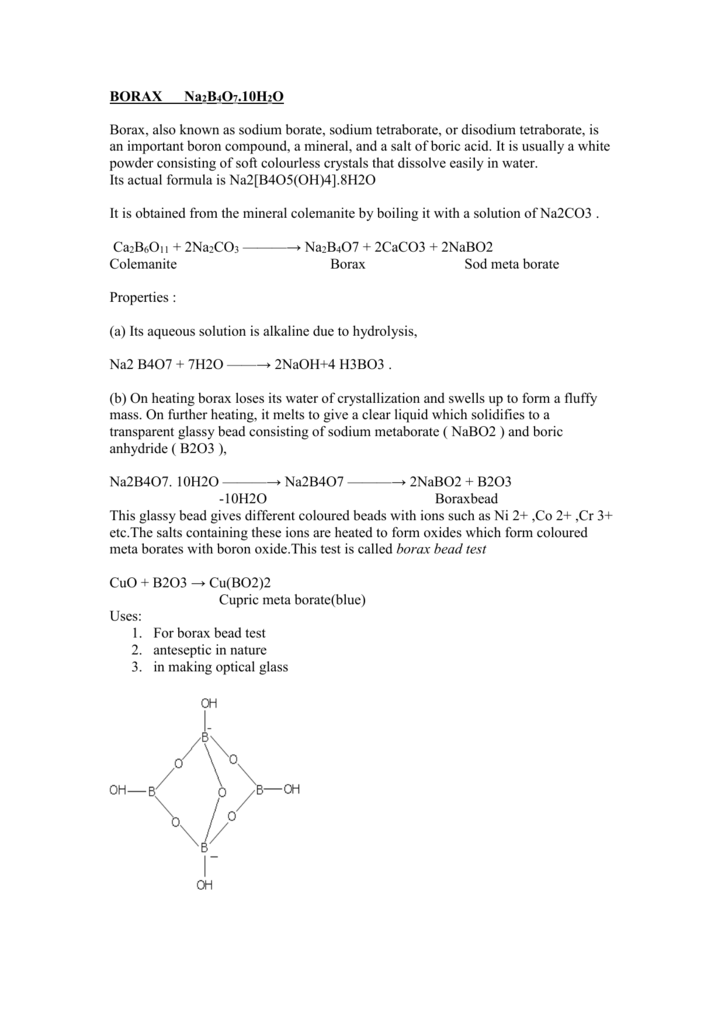
Borax (Na2B4O7•10H2O) || Properties || Structure & Hybridization || Preparation & Heating effect - YouTube

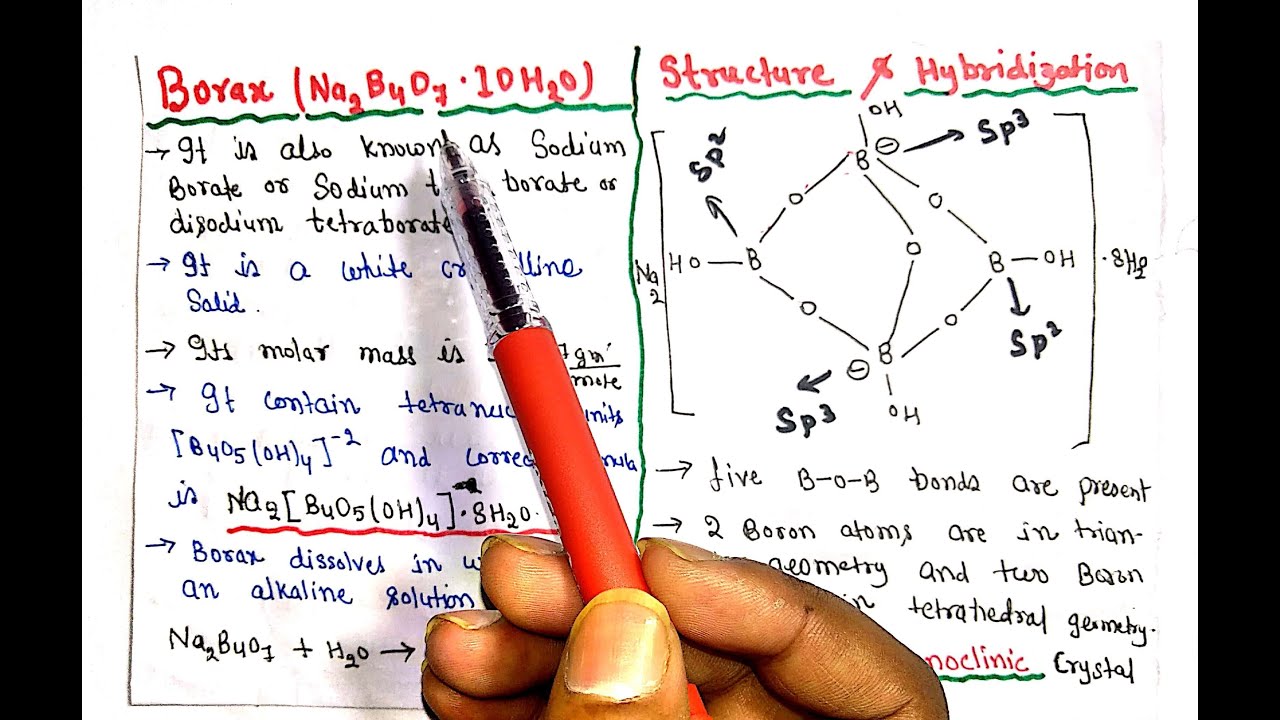
Borax has the formula Na2B4O7. 10H2O. It is a strong base in aqueous solution because OH^- ions are produced by reaction with water. (B4O7^2 - + 7H2O → 4H3BO3 + 2OH^-). How
![Analysis - Borax (Na2B4O7. 10H2O) ~2Na+ [B4O5(OH) 4] ²¯.8H2O No. of B—O—B bonds =5 No. Of B—O bonds =14 Oxidation state of B =+3 | Facebook Analysis - Borax (Na2B4O7. 10H2O) ~2Na+ [B4O5(OH) 4] ²¯.8H2O No. of B—O—B bonds =5 No. Of B—O bonds =14 Oxidation state of B =+3 | Facebook](https://lookaside.fbsbx.com/lookaside/crawler/media/?media_id=2274768172768993)
Analysis - Borax (Na2B4O7. 10H2O) ~2Na+ [B4O5(OH) 4] ²¯.8H2O No. of B—O—B bonds =5 No. Of B—O bonds =14 Oxidation state of B =+3 | Facebook

Sodium Borate, Decahydrate, Crystal, Reagent, ACS, 99.5-105%, Spectrum Chemical, Quantity: 500 g | Fisher Scientific

![Sodium Borate Decahydrate [Na2B4O7.10H2O] 99.9% ACS Grade 1.5 Lb in 3 Bottles | eBay Sodium Borate Decahydrate [Na2B4O7.10H2O] 99.9% ACS Grade 1.5 Lb in 3 Bottles | eBay](https://i.ebayimg.com/images/g/y~EAAOSw4UtWRVBY/s-l1600.jpg)





![Borax Decahydrate 10 Mol [Na2B4O7.10H2O] [CAS_1303-96-4] Technical Sta – Wintersun Chemical Borax Decahydrate 10 Mol [Na2B4O7.10H2O] [CAS_1303-96-4] Technical Sta – Wintersun Chemical](https://cdn.shopify.com/s/files/1/0724/7981/products/02-010-1.jpg?v=1532727515)