For one mole of a Van der Waals gas when b = 0 and T = 300 K, the PV vs 1V plot is shown above. The value of the Van der

How to Convert from Moles of One Substance to Moles of Another Substance (Stoichiometry part 1) - YouTube

Fly London MOL 2 Camel - Kostenloser Versand | Spartoo.de ! - Schuhe Klassische Stiefel Damen 159,20 €

The rate constant of a reaction is 9.2 × 10 5 mol 2 L 2 s 1. The order of the reaction is:A. 3B. 2C. ZeroD. 1

1 mole of N2 and 2 moles of H2 are allowed to react in a 1 dm^3 vessel. At equilibrium 0.8 mole of NH3 is formed. The concentration of H2 in the vessel is

SOLVED: Q9.A sample consisting of 2.00 mol He is expanded isothermally 22'C from 22.8 dm' to 31.7 dm' reversibly against constant external pressure equal to the final pressure of the gas (C)
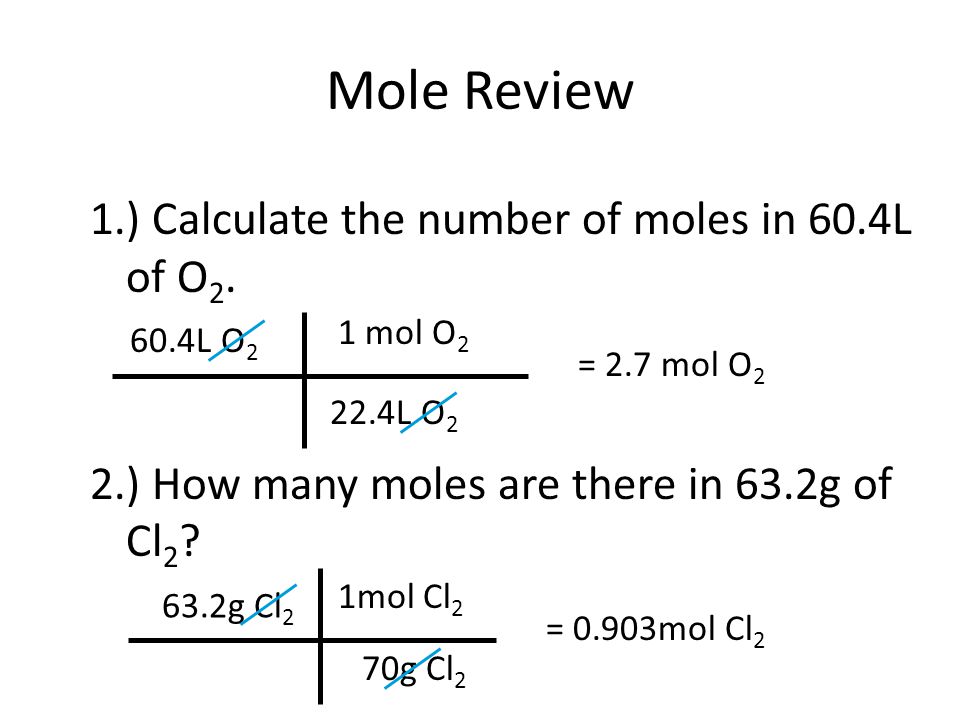
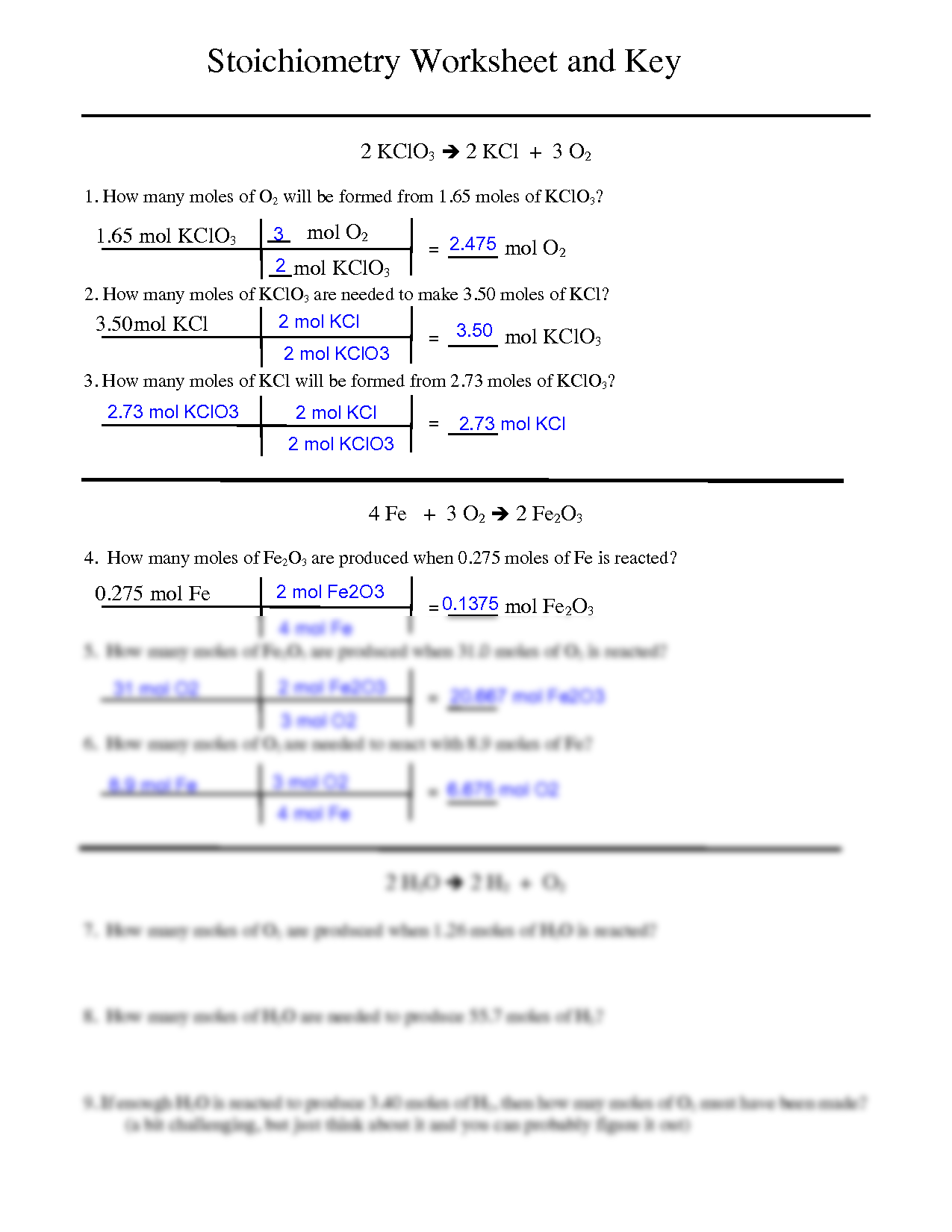
Mole Review 1.) Calculate the number of moles in 60.4L of O2. 2.) How many moles are there in 63.2g of Cl2? 1 mol O2 60.4L O2 = 2.7 mol O2 22.4L

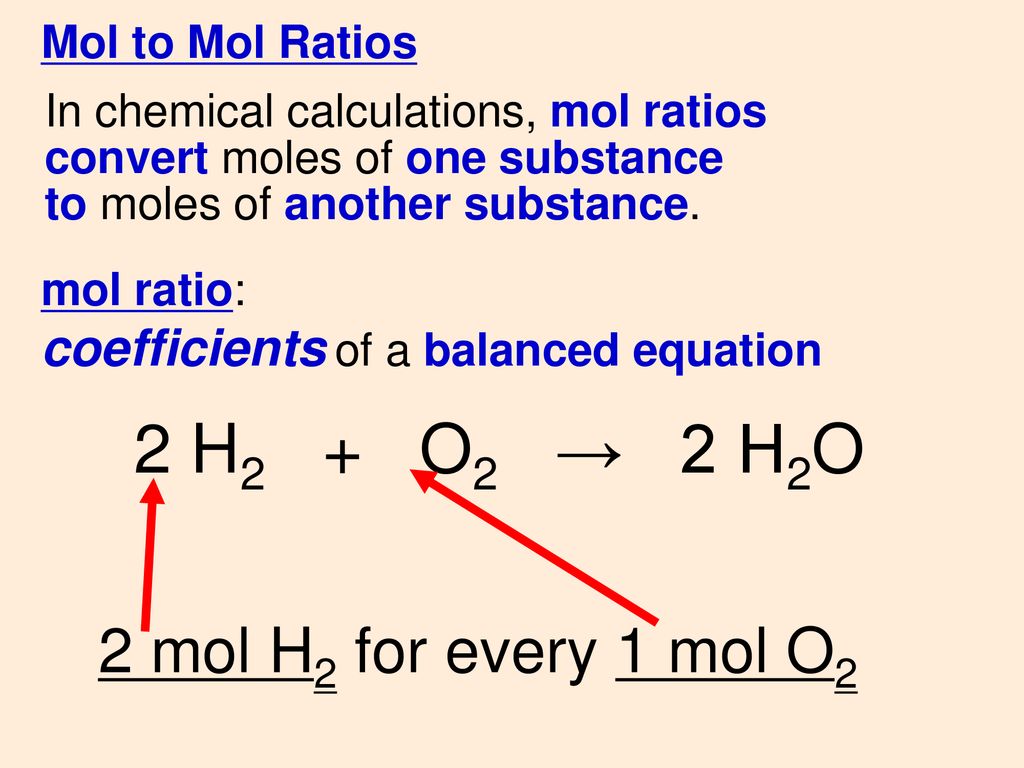
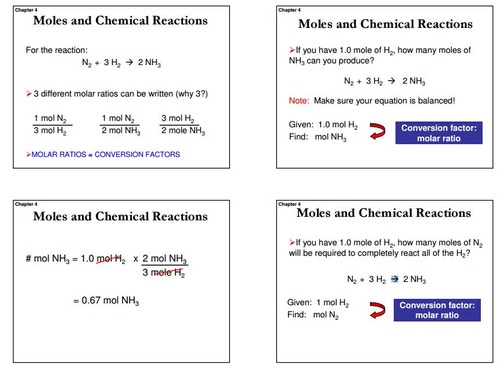
Mol ratio: coefficients of a balanced equation 2 H 2 + O 2 → 2 H 2 O 2 mol H 2 for every 1 mol O 2 In chemical calculations, mol ratios convert moles of. - ppt download