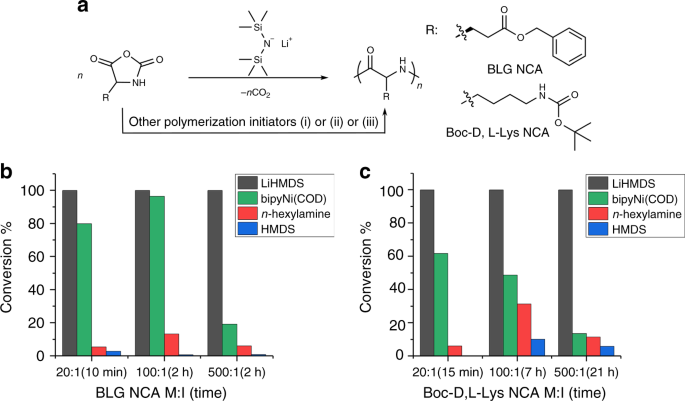
Lithium hexamethyldisilazide initiated superfast ring opening polymerization of alpha-amino acid N-carboxyanhydrides | Nature Communications

LiHMDS‐Mediated Deprotonative Coupling of Toluenes with Ketones - Shigeno - Chemistry – A European Journal - Wiley Online Library
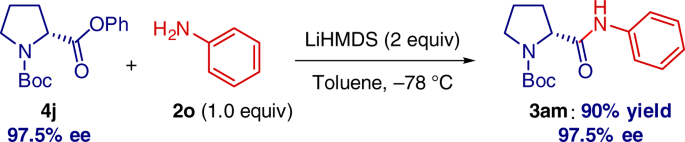
Highly selective transition-metal-free transamidation of amides and amidation of esters at room temperature | Nature Communications
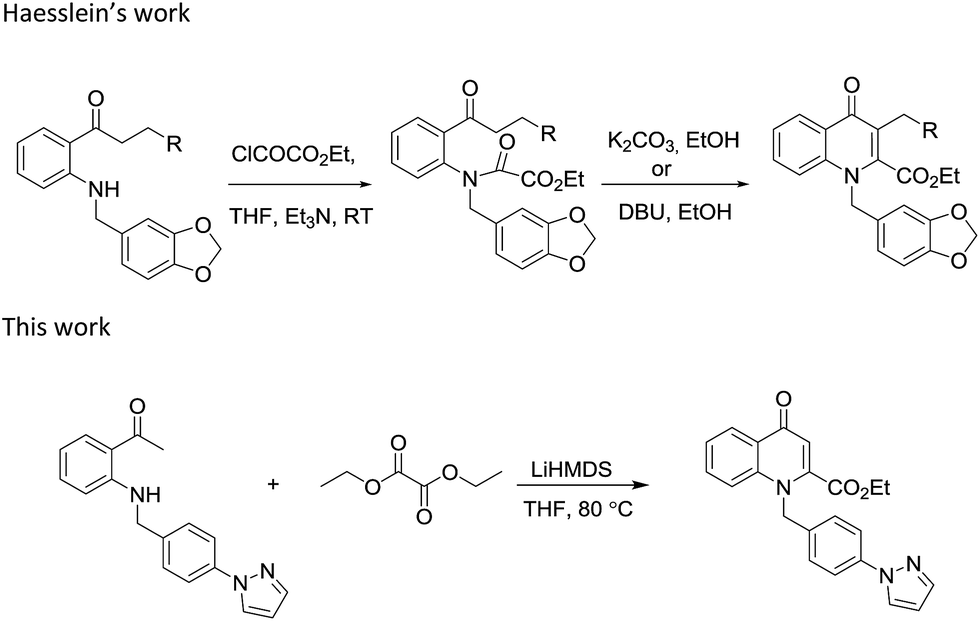
Efficient synthesis of novel N -substituted 2-carboxy-4-quinolones via lithium bis(trimethylsilyl)amide (LiHMDS)-induced in situ cyclocondensation rea ... - RSC Advances (RSC Publishing) DOI:10.1039/C6RA28631C

Reaction of Ketones with Lithium Hexamethyldisilazide: Competitive Enolizations and 1,2-Additions | Journal of the American Chemical Society
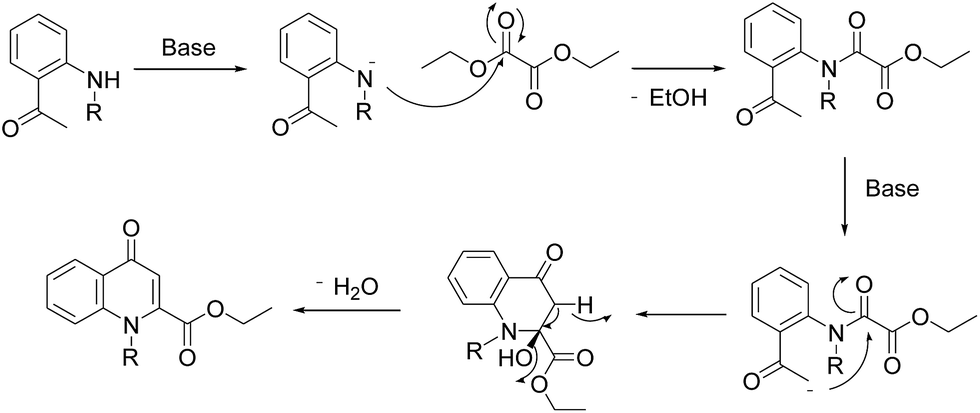
Efficient synthesis of novel N -substituted 2-carboxy-4-quinolones via lithium bis(trimethylsilyl)amide (LiHMDS)-induced in situ cyclocondensation rea ... - RSC Advances (RSC Publishing) DOI:10.1039/C6RA28631C

Lithium Hexamethyldisilazide-Mediated Enolization of Highly Substituted Aryl Ketones: Structural and Mechanistic Basis of the E/Z Selectivities. - Abstract - Europe PMC
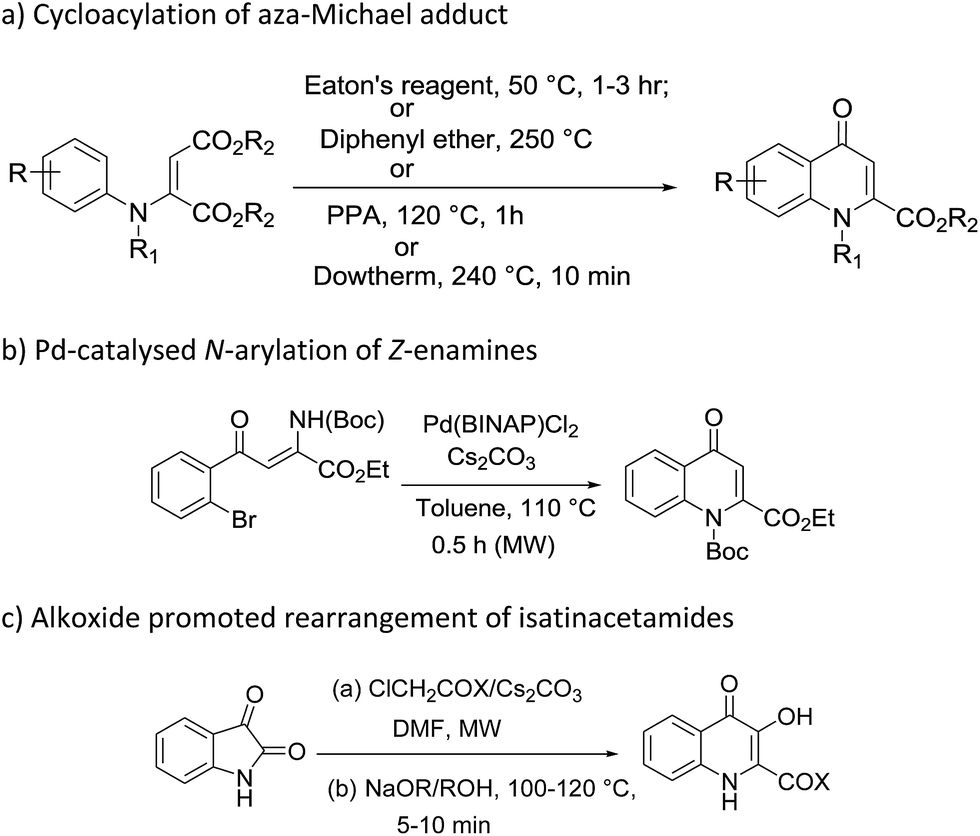
Efficient synthesis of novel N -substituted 2-carboxy-4-quinolones via lithium bis(trimethylsilyl)amide (LiHMDS)-induced in situ cyclocondensation rea ... - RSC Advances (RSC Publishing) DOI:10.1039/C6RA28631C

LiHMDS: Facile, highly efficient and metal-free transesterification under solvent-free condition - ScienceDirect

LiHMDS: Facile, highly efficient and metal-free transesterification under solvent-free condition - ScienceDirect
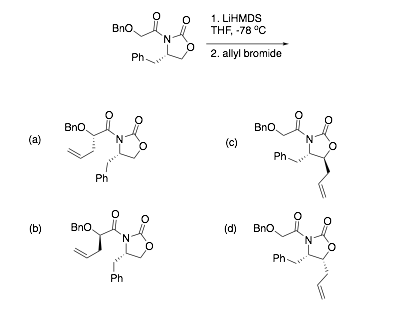
Lithium Hexamethyldisilazide-Mediated Enolization of Acylated Oxazolidinones: Solvent, Cosolvent, and Isotope Effects on Competi

Lithium hexamethyldisilazide-mediated enolizations: influence of triethylamine on E/Z selectivities and enolate reactivities. - Abstract - Europe PMC