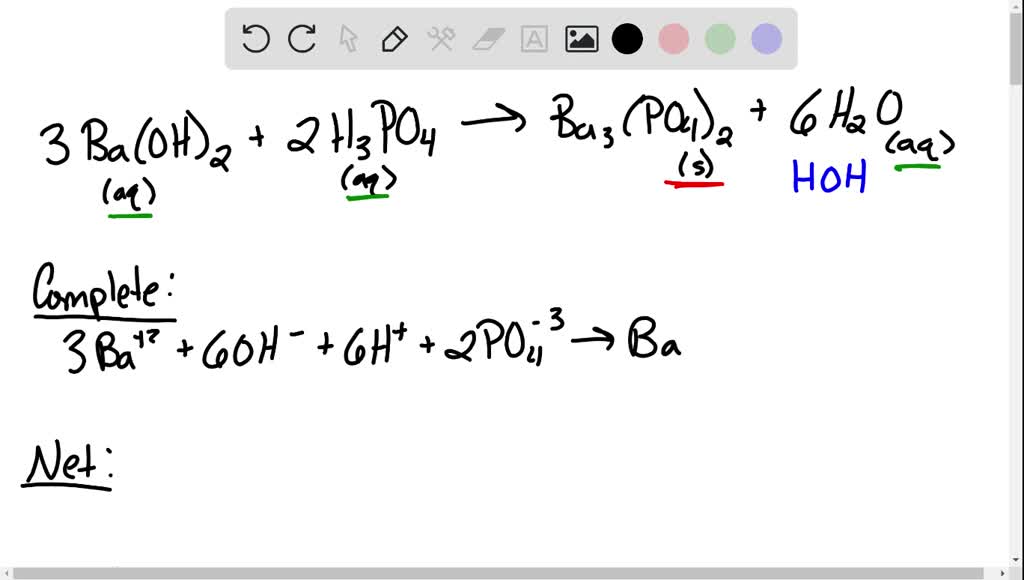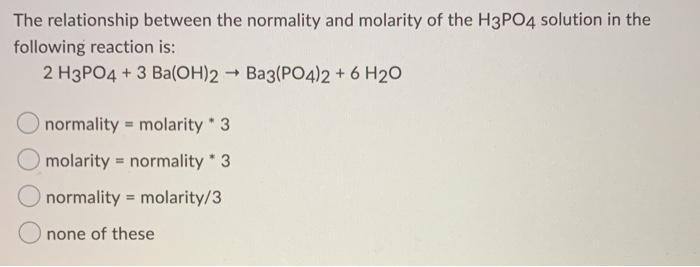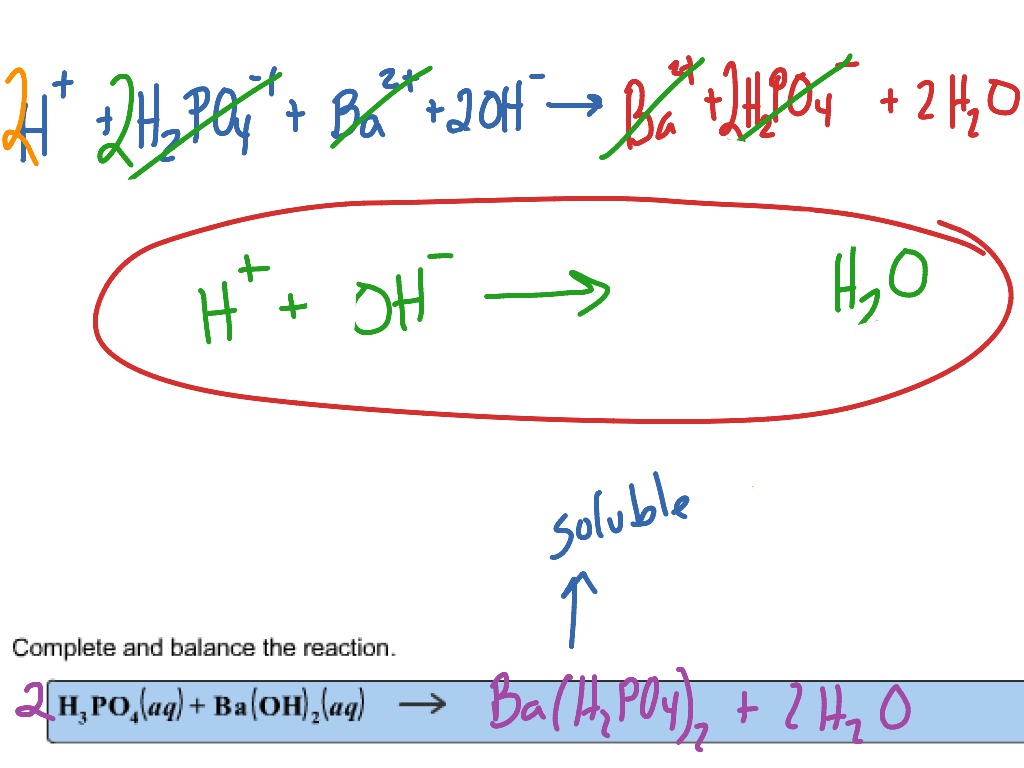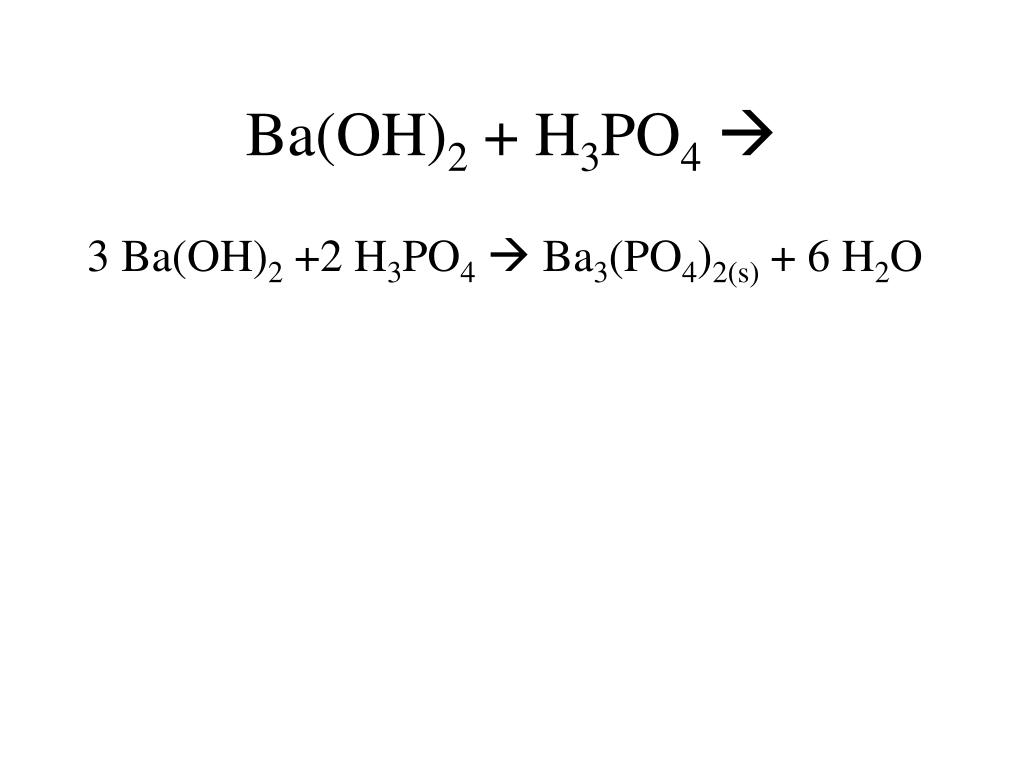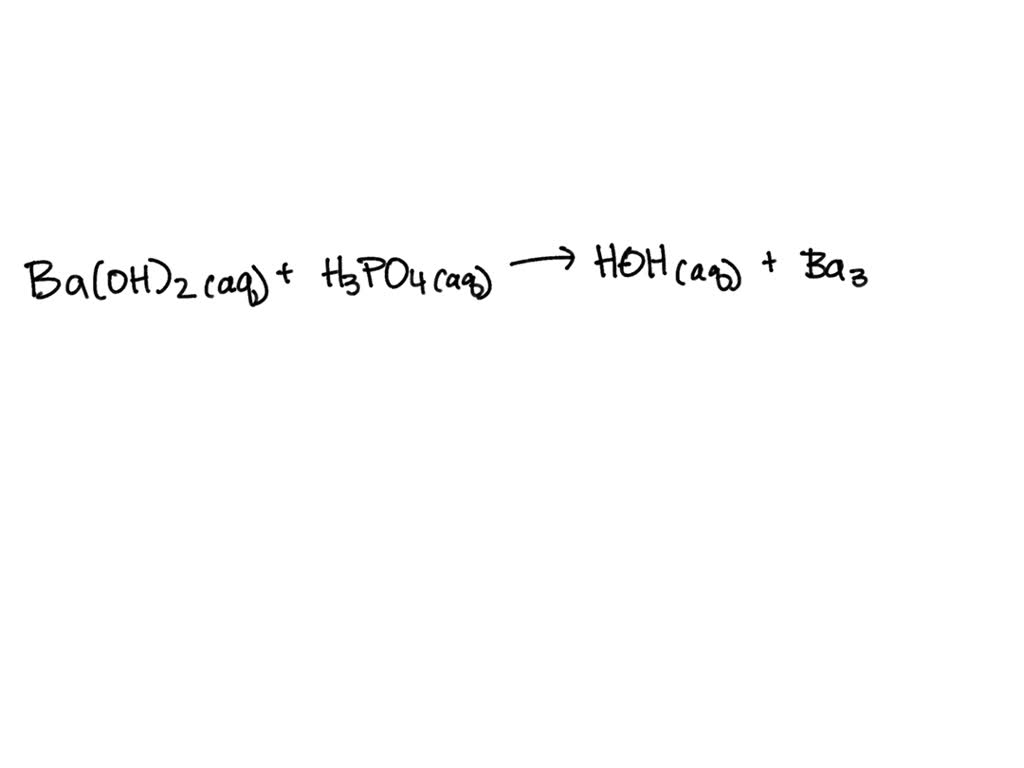
SOLVED: When a solution containing barium hydroxide is added to a solution containing phosphoric acid (HaPO4), a white precipitate forms and settles to the bottom of the beaker: What is the white
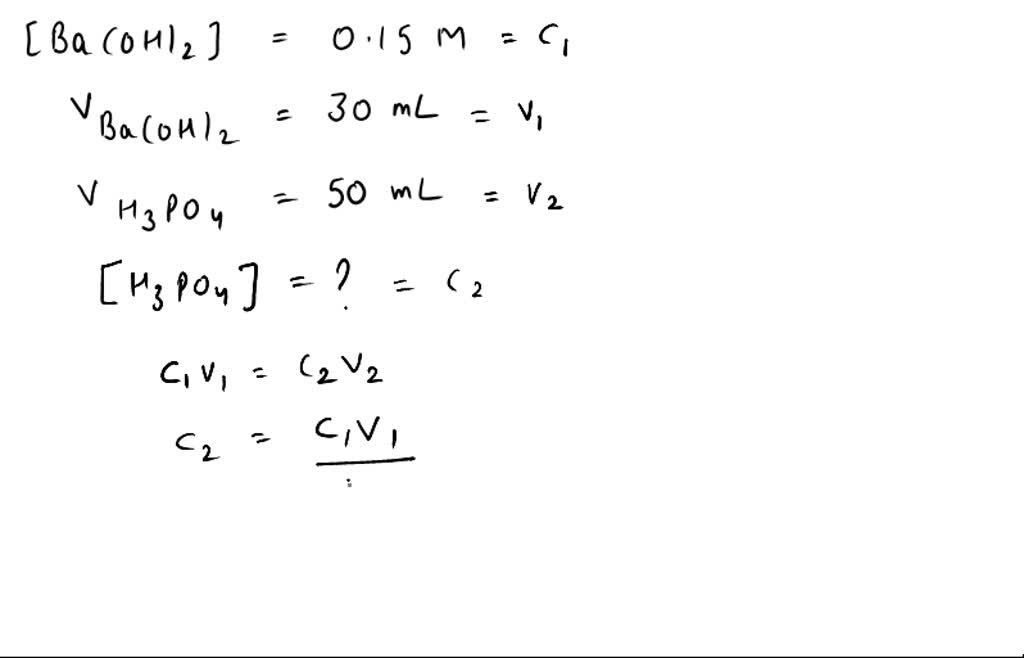
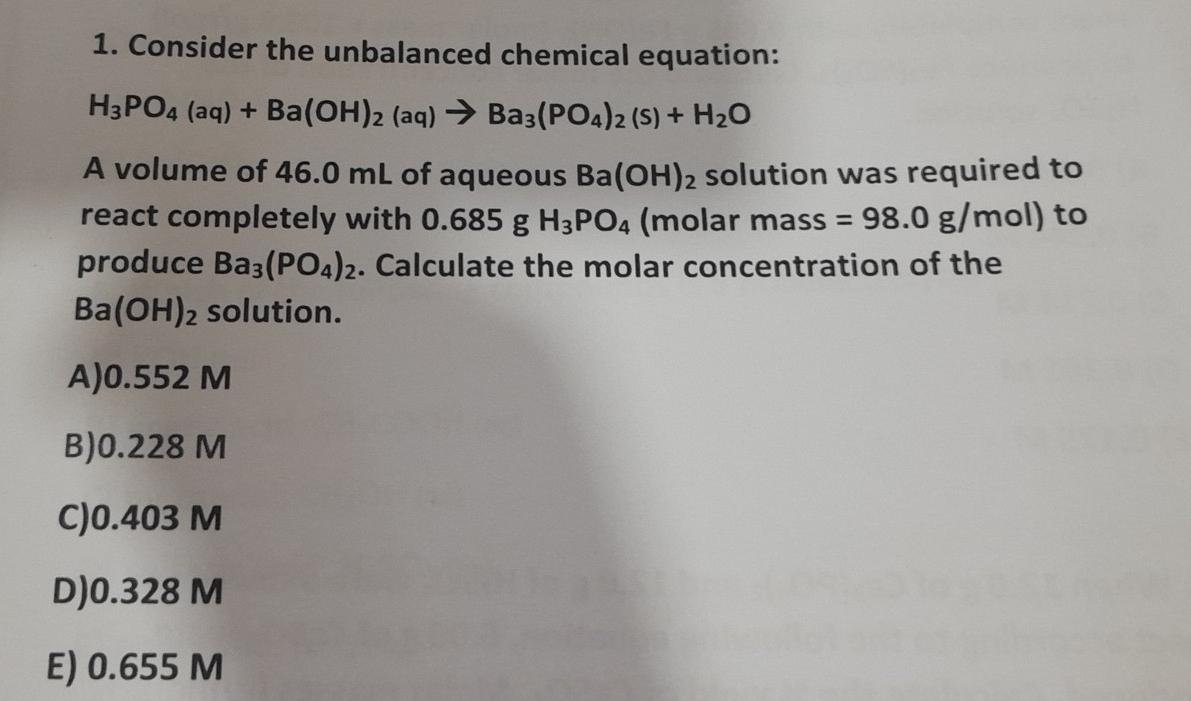
SOLVED: If 30.0 mL of 0.15 M Ba(OH)2 was needed to neutralize 50.0 mL of an H3PO4 solution. What is the concentration of the original H3PO4 solution?

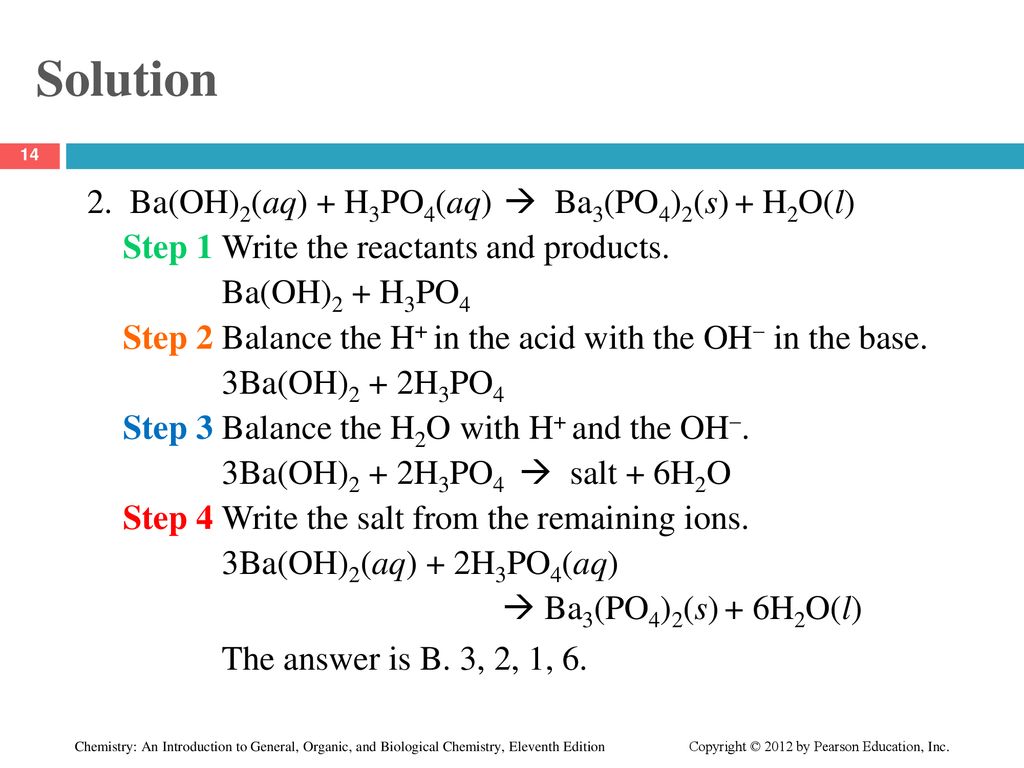
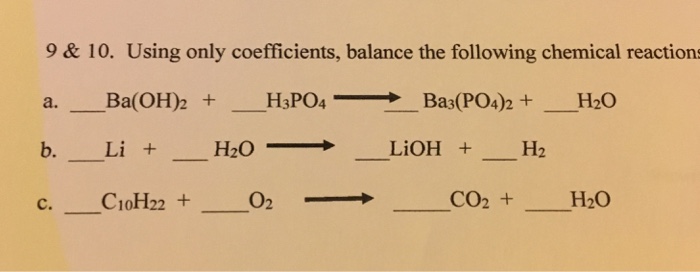
SOLVED: Aqueous solutions of barium hydroxide (Ba(OH)2) and phosphoric acid (H3PO4) will react to yield barium phosphate and water. In the balanced equation for this reaction, what is the lowest possible whole

SOLVED: If 30.0 mL of 0.15 M Ba(OH)2 was needed to neutralize 50.0 mL of an H3PO4solution. What is the concentration of the original H3PO4 solution?
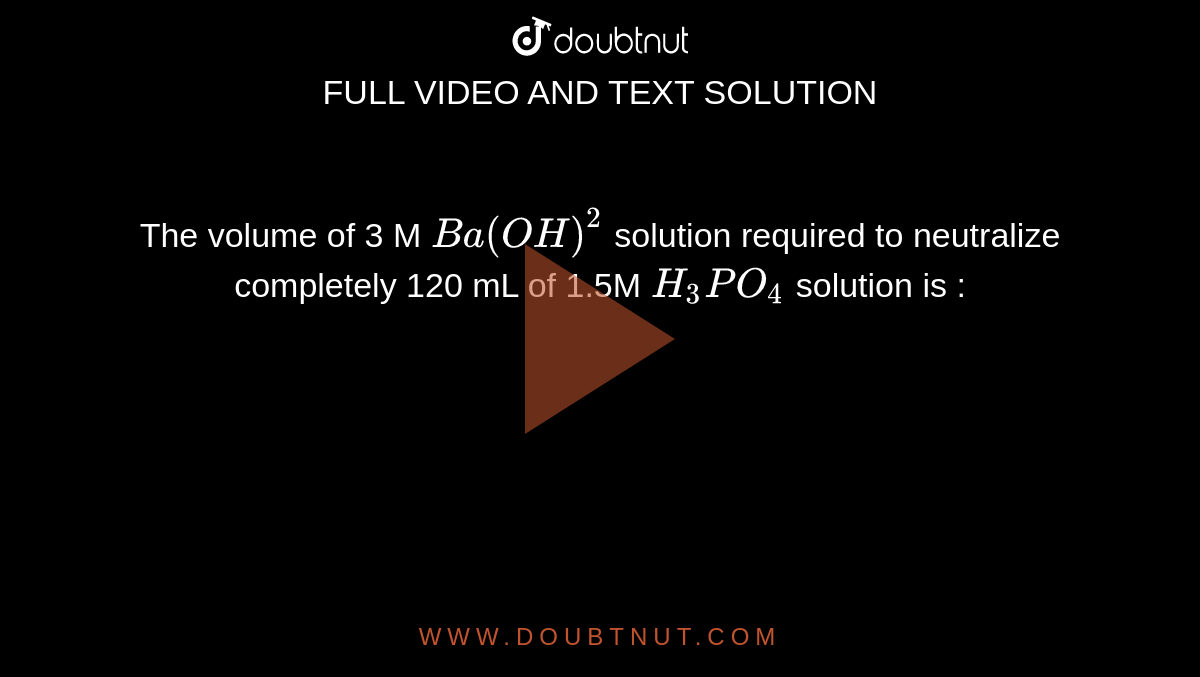
The volume of 3 M Ba(OH)^(2) solution required to neutralize completely 120 mL of 1.5M H(3)PO(4) solution is :

SOLVED: Barium hydroxide and phosphoric acid react as follows: 3 Ba(OH)2(s) + 2 H3PO4(aq) –> Ba3(PO4)2(aq) + 6 H2O(l) If 39.5 g of Ba(OH)2 are allowed to react with 51.0 g of