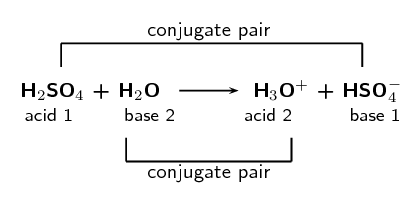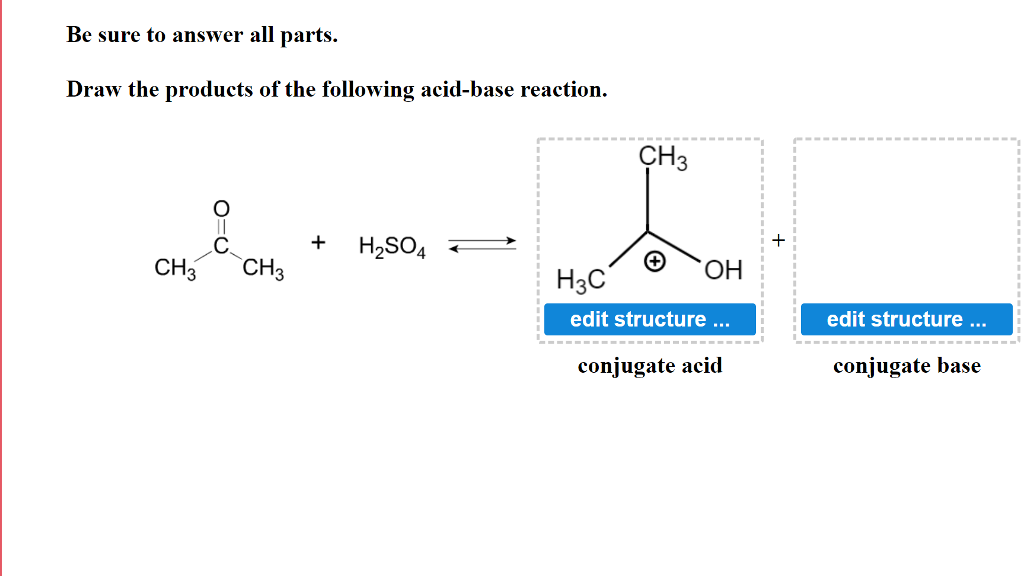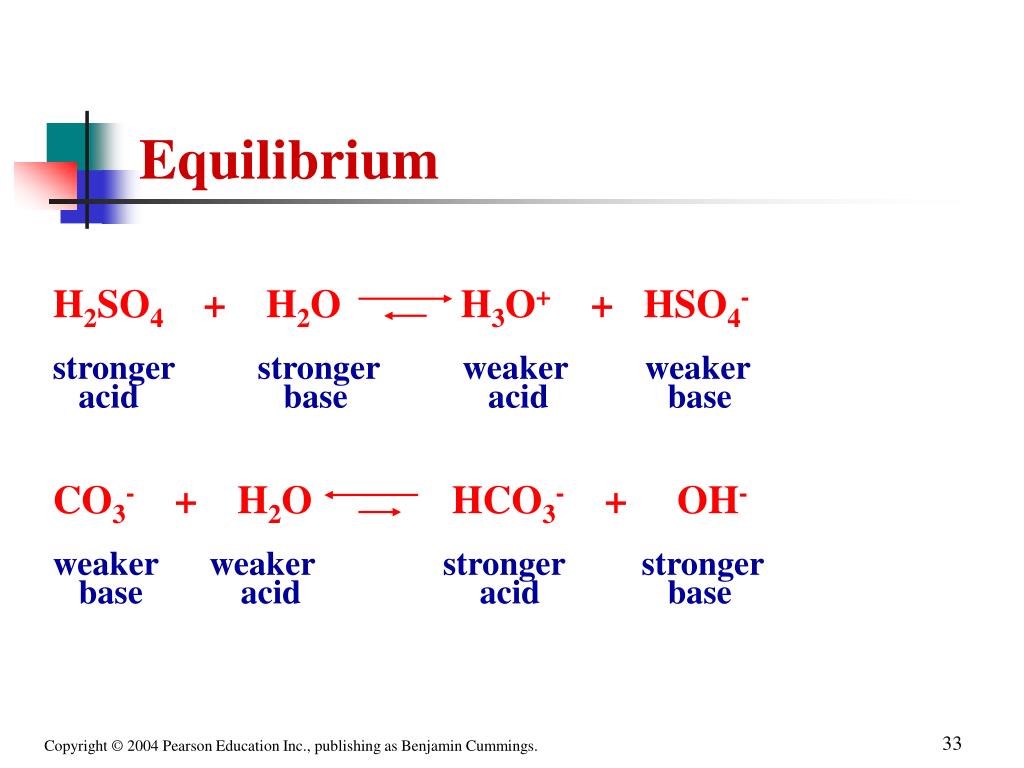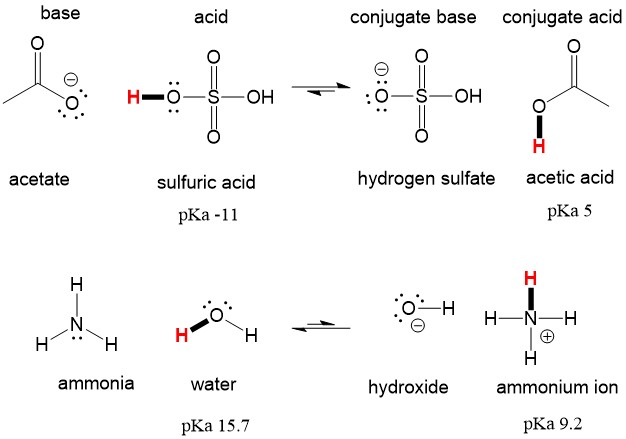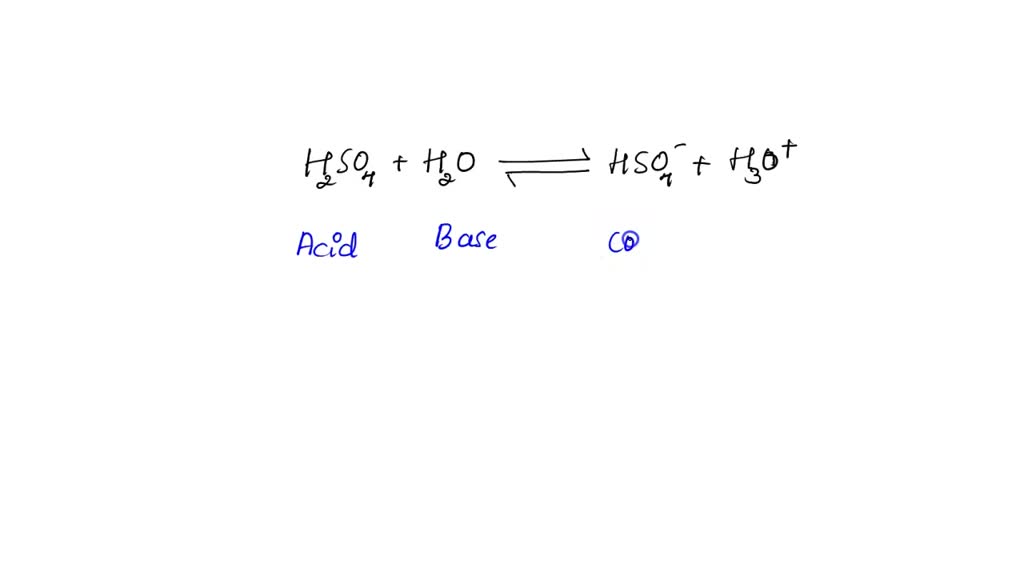
SOLVED: In the following equation, identify the Bronsted Lowry conjugate base H2SO4 + H2O <-> HSO4 - + H3O+ Group of answer choices H2SO4 HSO4- H2O H3O+

Illustration of the initial acid‐base clustering in urban Beijing. (a)... | Download Scientific Diagram

Identify the acid-conjugate base pair in this balanced equation: H2SO4 + 2NaOH → 2H2O + - Brainly.com
✓ Solved: Hydrazine, N2H4, is a weak base and can react with an acid such as sulfuric acid: 2N2H4(aq)+H2SO4(aq)→...