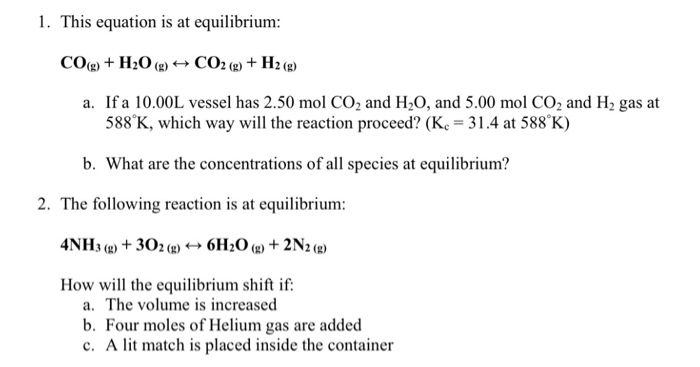
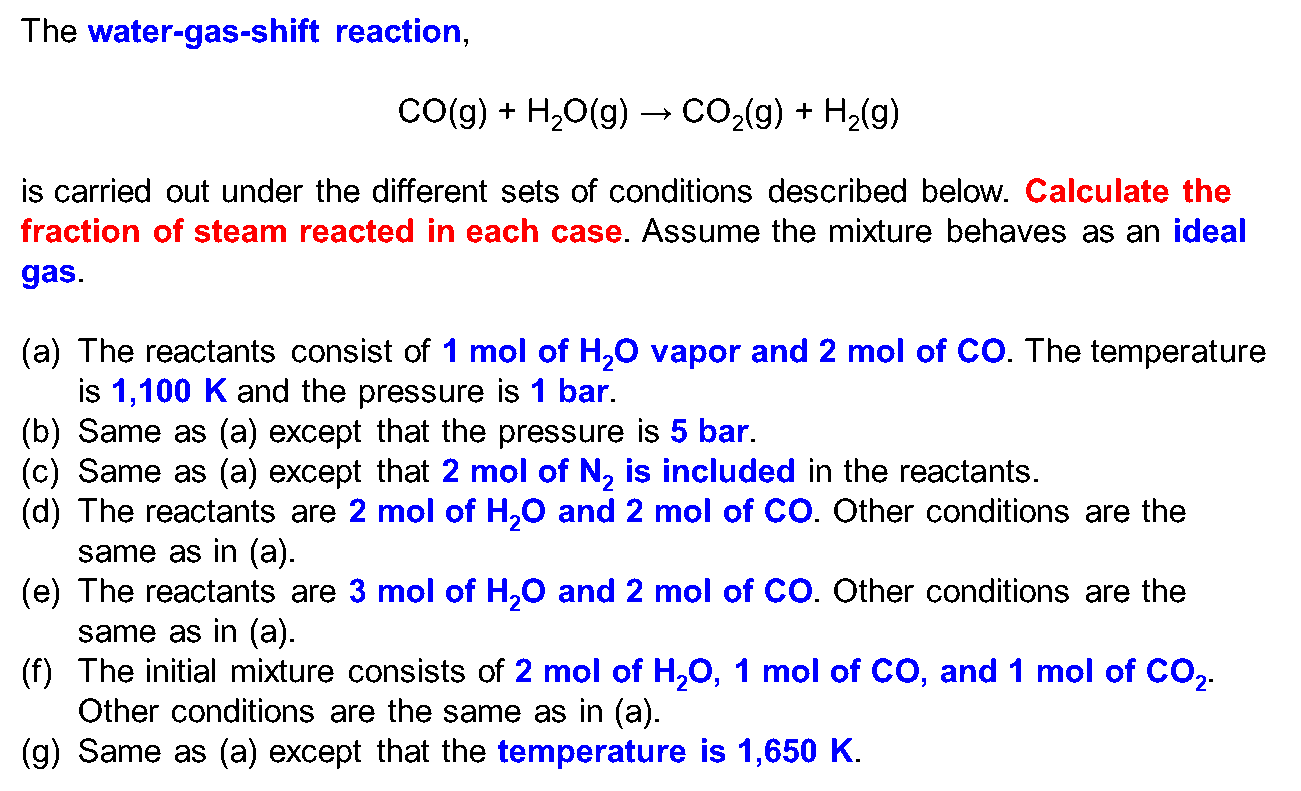
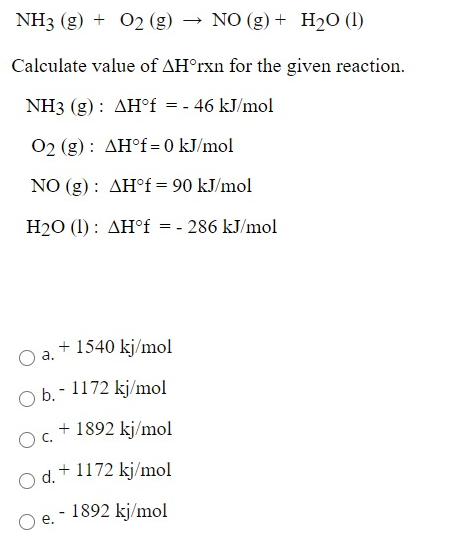
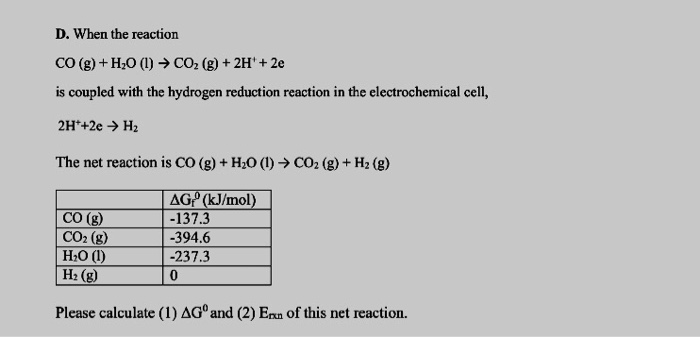
0.262g of a subs†an ce gave on combustion 0.361g of co2 and 0.14g H2O what the emperical formla of substance

OneClass: 4) For the reaction H2(g) + CO2(g) H2O(g) + CO(g) at 700°C, Kp = 0.534. Calculate the numb...
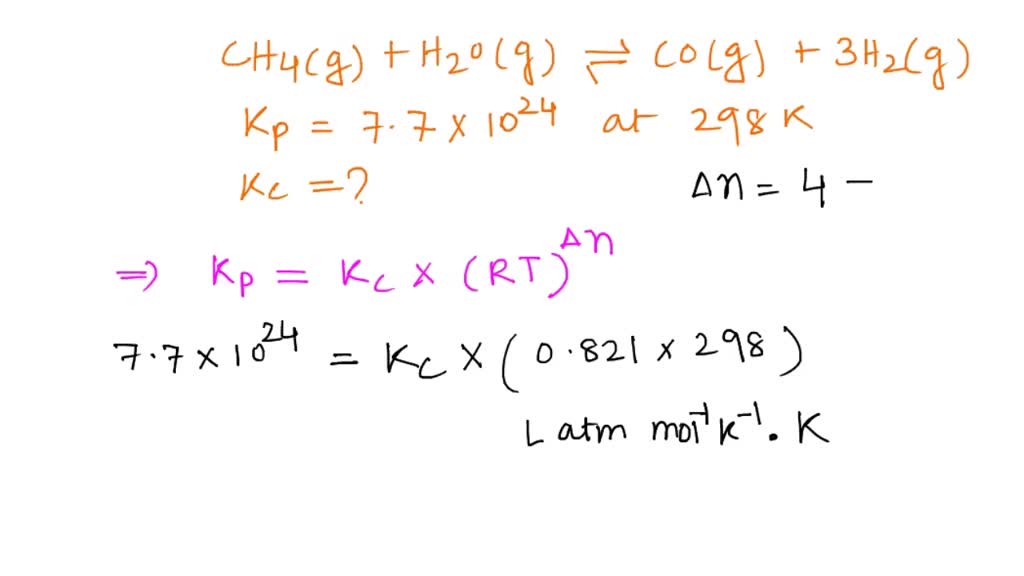
SOLVED: Consider the reaction below. H2O (g) + CH4 (g) <—> CO (g) + 3H2 (g) Kc = 4.7 at 1400 K What is Kp for this reaction at 1400 K? 6.2 x 104 4.7 8.2 x 10^8

Consider the following reaction: H2O(l)→H2O(g);ΔH1 = 44kJ 2CH3OH(l) + 3O2→ 4H2O(l) + 2CO2(g) ; ΔH2 = - 1453kJ What is the value of Δ H for the second reaction if water vapor