PDF) Photoionization and ab initio study of Ba(H2O)n (n = 1-4) clusters | Carlos J Cobos - Academia.edu

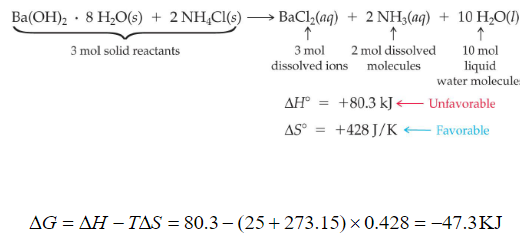
OneClass: 3. Consider the reaction: 2HCl(aq) + Ba(OH)2(a) â†' BaCl2(aq) + 2 H2O(l) Δ --118 kl A) Cal...

рассчитайте по термохимическому уравнению BaO+H2O=Ba(OH)2+73 кДж сколько выделяется энергии - Школьные Знания.com
40. 50ml H2O is added to a 50ml solution of Ba(OH)2 of strenth 0.01M. The pH value of the resuultingsolution will be
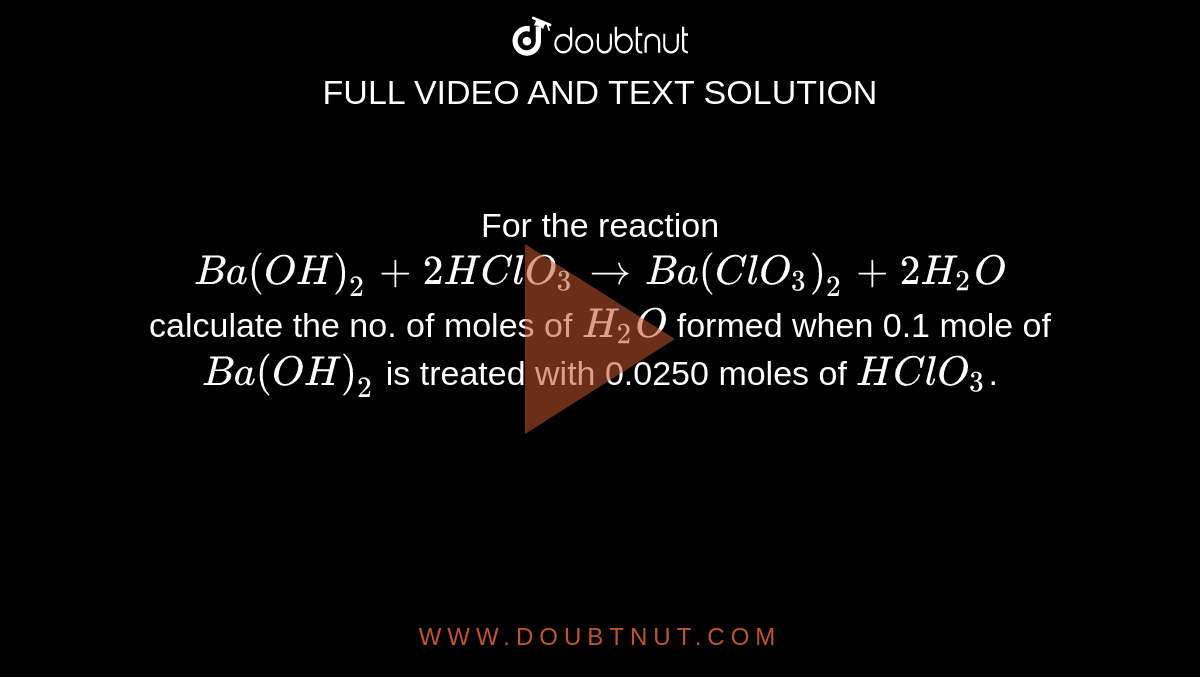
For the reaction Ba(OH)(2)+2HClO(3)rarr Ba(ClO(3))(2)+2H(2)O calculate the no. of moles of H(2)O formed when 0.1 mole of Ba(OH)(2) is treated with 0.0250 moles of HClO(3).