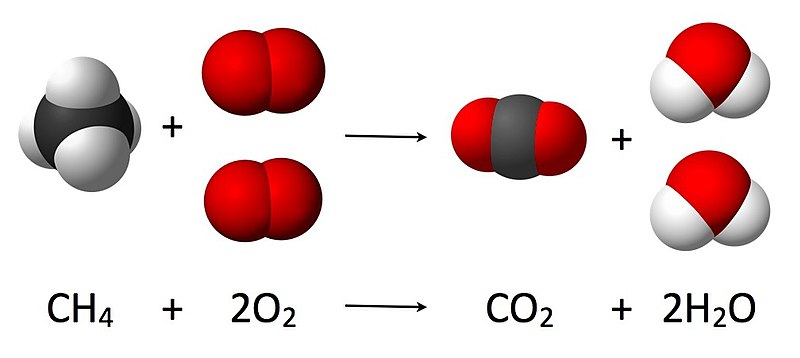
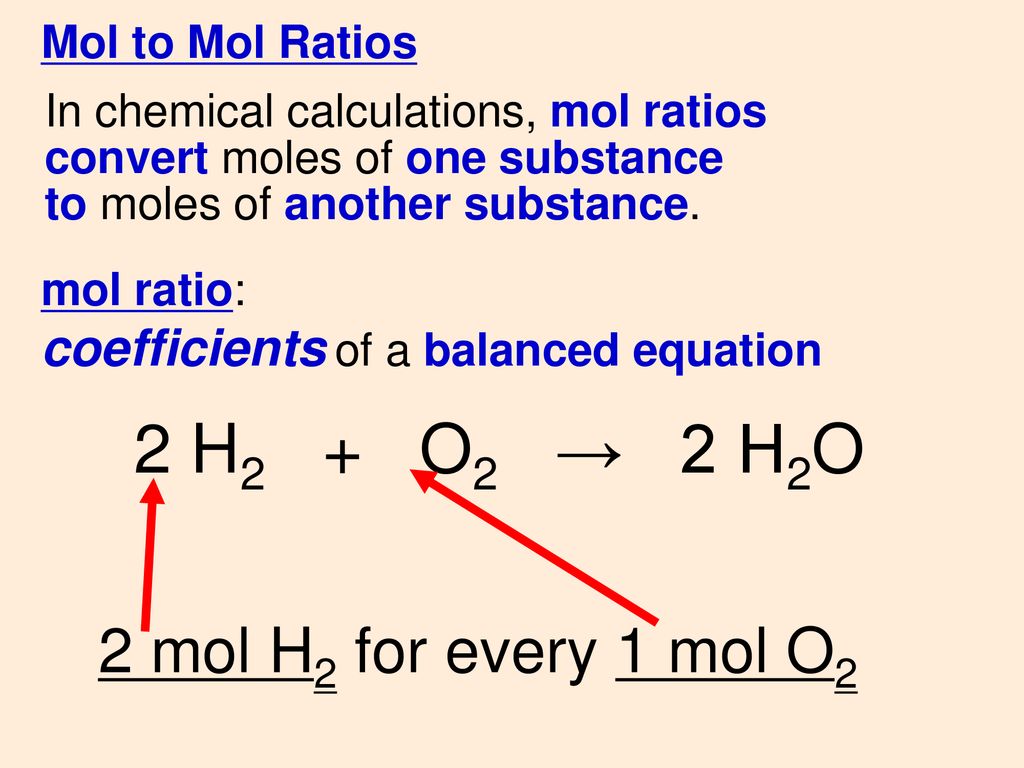
Chemical Structure H2o And 2h2o Stock Photo - Download Image Now - 2015, Chemical Formula, Chemistry - iStock

Overall reaction of Cathode and Anode: Cathode: 2 H2O(l) + 2e− → H2(g)... | Download Scientific Diagram
![Milde Hydrothermalsynthese von Na-Cancrinit Na8[AlSiO4]6(NO3)2(H2O)4 bei 60 GradC und Untersuchungen zur thermischen Stabilität (kartoniertes Buch) | Bessunger Buchladen Milde Hydrothermalsynthese von Na-Cancrinit Na8[AlSiO4]6(NO3)2(H2O)4 bei 60 GradC und Untersuchungen zur thermischen Stabilität (kartoniertes Buch) | Bessunger Buchladen](https://medien.umbreitkatalog.de/bildzentrale_original/978/374/480/0952.jpg)
Milde Hydrothermalsynthese von Na-Cancrinit Na8[AlSiO4]6(NO3)2(H2O)4 bei 60 GradC und Untersuchungen zur thermischen Stabilität (kartoniertes Buch) | Bessunger Buchladen
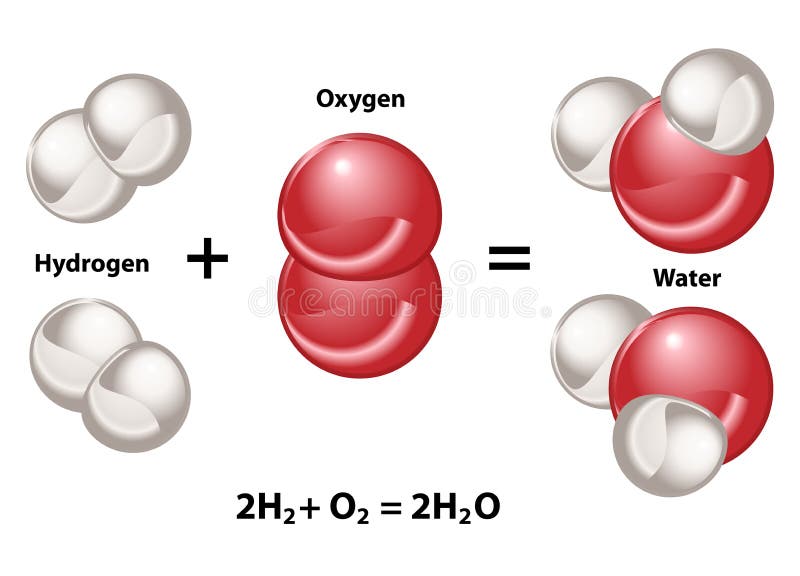
Molecular Compound in H2O Water Molecule Stock Vector - Illustration of creating, compounds: 195911340

Complete the following equation: Na2O2 + 2H2O → (W) + H2O2 2KO2 + 2H2O→ (X) + (Y) + O2 Na2O + CO2→ (Z)

Synthesis of cis-Cu(gly)2·H2O, trans-Cu(gly)2, and cis-Ni(gly)2(H2O)2 and their Characterization Using Thermal and Spectroscopic Techniques—A Capstone Inorganic Laboratory | Journal of Chemical Education

Complete the following statements for the complex ion (Co(en)2(H2O)CN)2+. a. "en" is the abbreviation for. b. The oxidation number of Co is. c. The coordination number of Co is. d. is a